

Avogadro and the Mole
Sometimes grasping the concept of the mole can be a challenge for some students. Initially, even the name of the chemist who developed the idea of the mole is a little "strange." To make the presentation less daunting, I utilize an avocado to help students learn the pronunciation of Avogadro's name. Of course its not the same, but likening Avogadro to avocado makes learning his name less intimidating. After showing them the avocado, pass it around and then if you have more on hand, consider making some dip for snacktime!

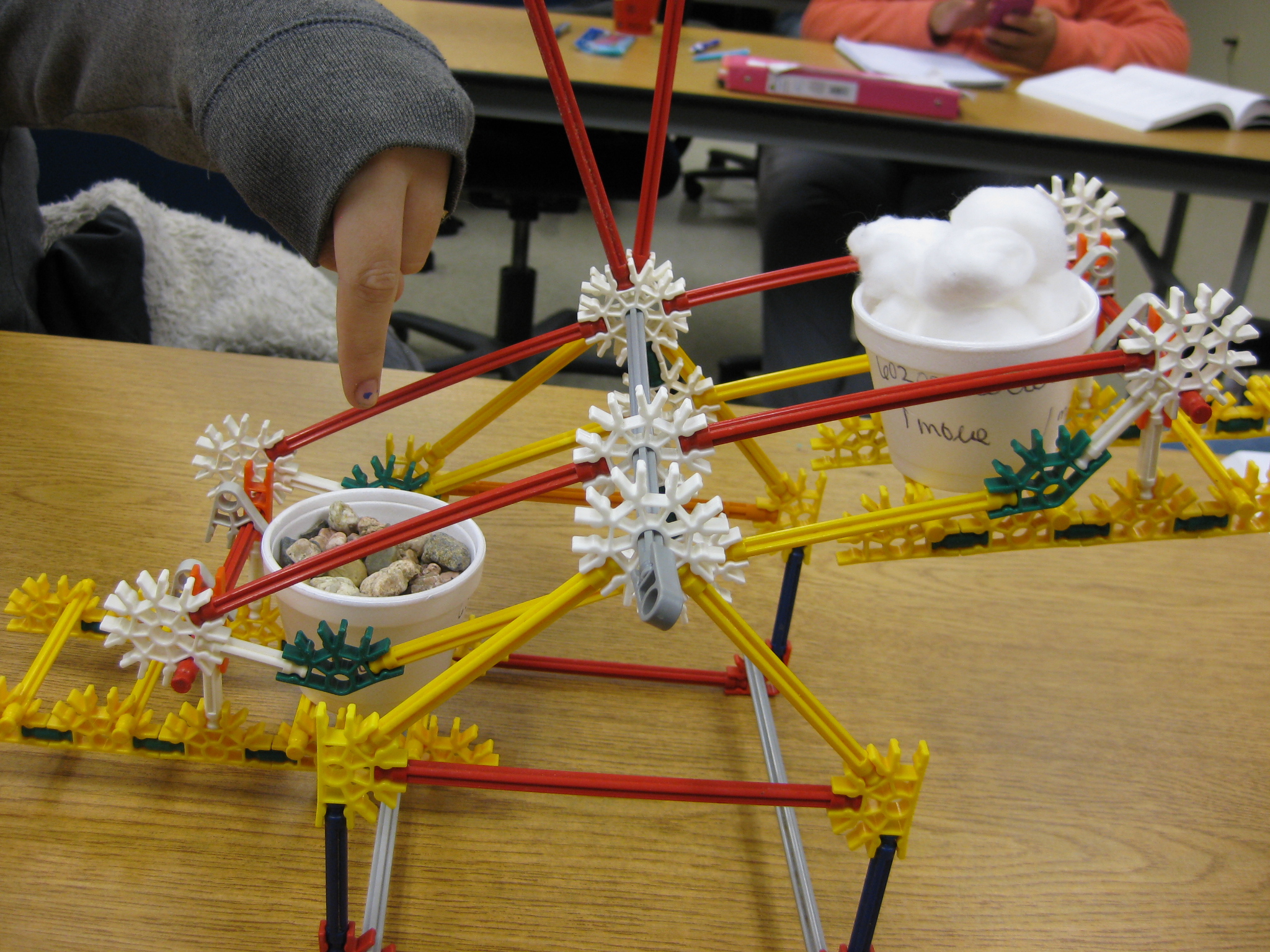
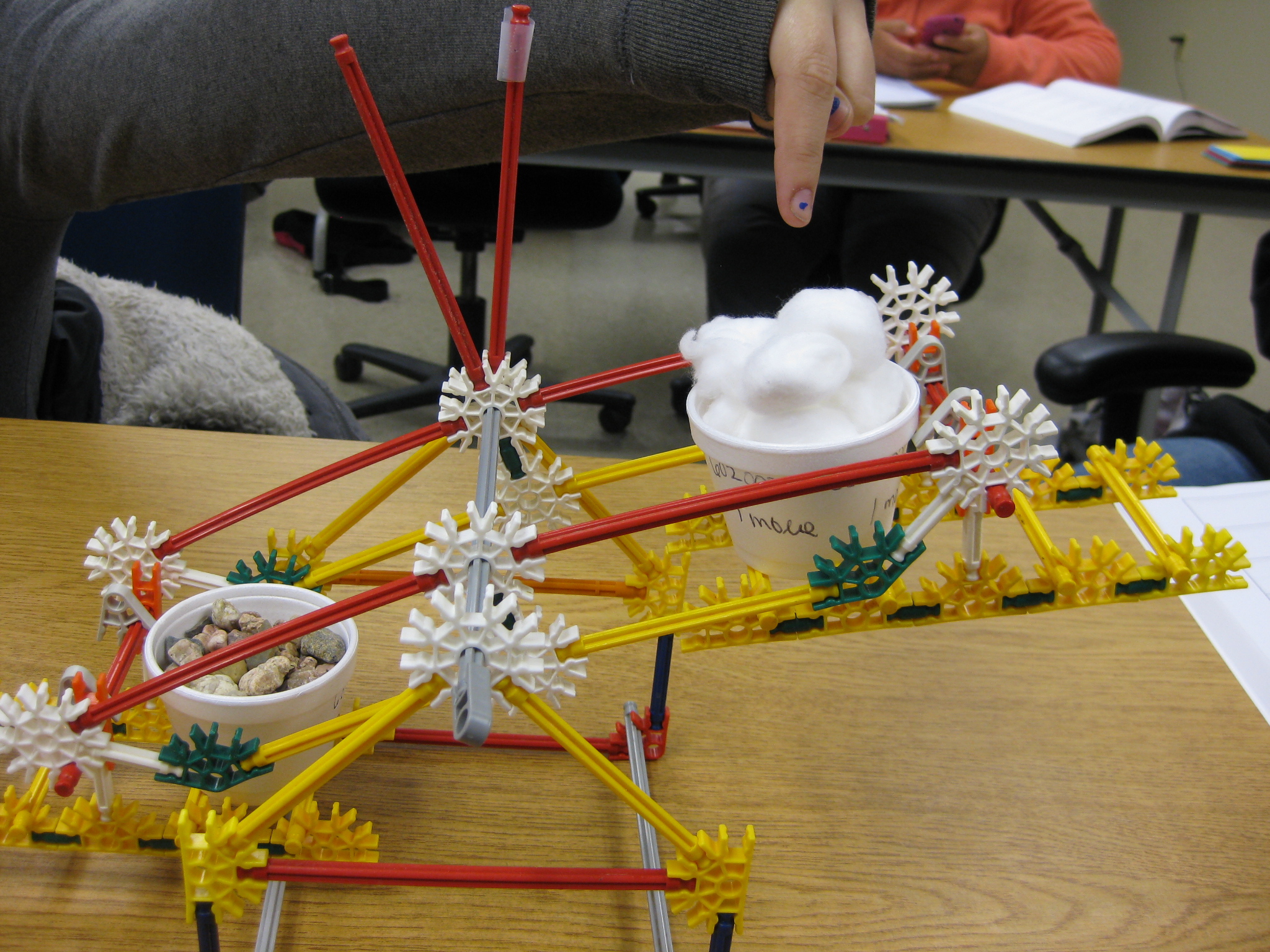
Then, to present the mole concept, I give each student two styrofoam cups. On each cup I ask them to write "1 mole" several times all around the rim of the cup. Next, I have them fill one cup with cotton balls and then fill the other with gravel (it doesn't have to be gravel; anything denser than cotton balls will work such as beans, sand, etc.). Have them fill each cup to the top, but to not necessarily smash down the cotton balls.
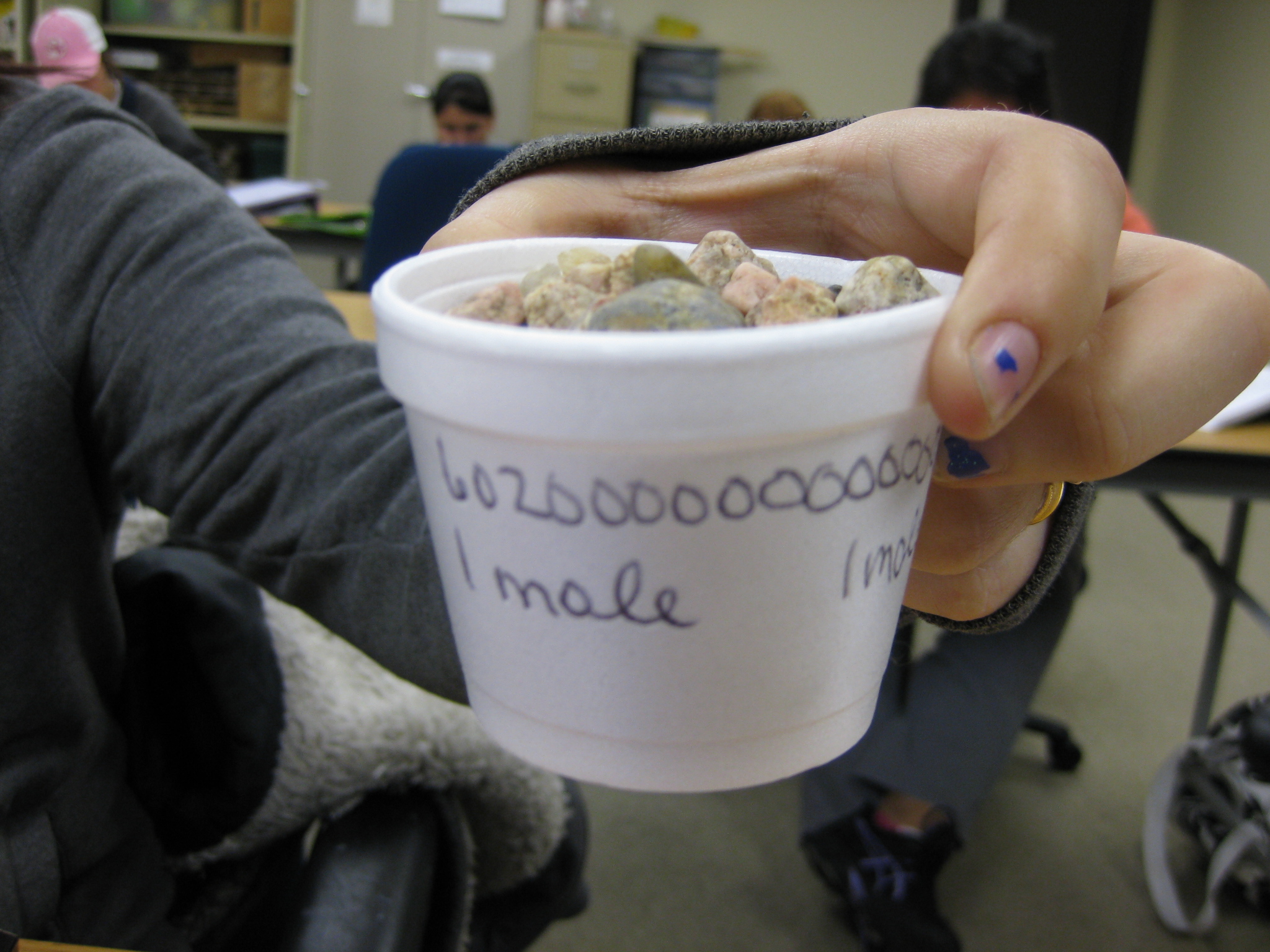

Then, I tell them that when each cup is full, it contains 1 mole of that substance. Note that the cup is not really a mole of that substance, but that it "represents" 1 mole of that substance. I liken the mole measure to being similar as a measuring cup; its an arbitrary amount that we agree upon. I have them hold-up one mole of gravel and then hold-up one mole of the cotton balls. It is important for them to realize that regardless of what is inside the cup, each cup holds 1 mole of that substance.
Next, I introduce the idea of Avogadro's number. First, I write it on the board in scientifice notation (6.02 x 1023 atoms.) Then, I write it in the "long" form (602,000,000,000,000,000,000,000 - twenty-one zeros, right?)
I now ask my students to write Avogadro's number on each of their styrofoam cups; have them right both the scientific notation form and the "long" form.

Then, I emphazise that 1 mole is equal to 6.02 x 1023 atoms. I ask them to hold up their cup of gravel and ask them how many moles are in the cup. They should respond with "one." I then ask them how many atoms of gravel are in the cup. They should respond with "6.02 x 1023 atoms." Next, I ask them to hold-up their cup of cotton balls and again I ask how many moles are in the cup. They should respond with one. Again, I ask how many atoms are in the cup if one mole is in the cup. They should respond with "6.02 x 1023 atoms."
The next question is, "Do all moles of substances weigh the same amount?" Your students should readily respond with, "No, they don't weigh the same!" Have them hold up both cups simultaneously and compare.

If you have your charge balance handy or another set of scales, demonstrate that while we each have one mole of each subtance which is 6.02 x 1023 atoms, moles of varying substances have their own weight or mass.
Then, I refer them to their periodic tables and introduce the atomic mass numbers. I tell them that it's the atomic mass value which tells how much one mole of that element weighs (in grams.)
I then ask them if they can guess why masses of moles of the elements varies. Point out how the mass value increases as you go across each period of elements. Hopefully, someone will infer that it's the increasing number of "heavy" protons and neutrons present in atoms of each element which contribute to the increasing atomic mass values (refer back to cannon ball and ping pong ball chart.) I then ask if any students have had experiences with carrying car batteries. I ask them if they know what is inside the battery (lead and sulfuric acid.) I focus on the lead component and find it on the periodic table and point out it's LARGE atomic mass value. I compare it to the element, aluminum. Many will have had experiences with aluminum cans and know they are very lightweight. Refer back to the styrofoam cups and ask them which cup could represent the mole of lead (hopefully they'll respond with cup of gravel).
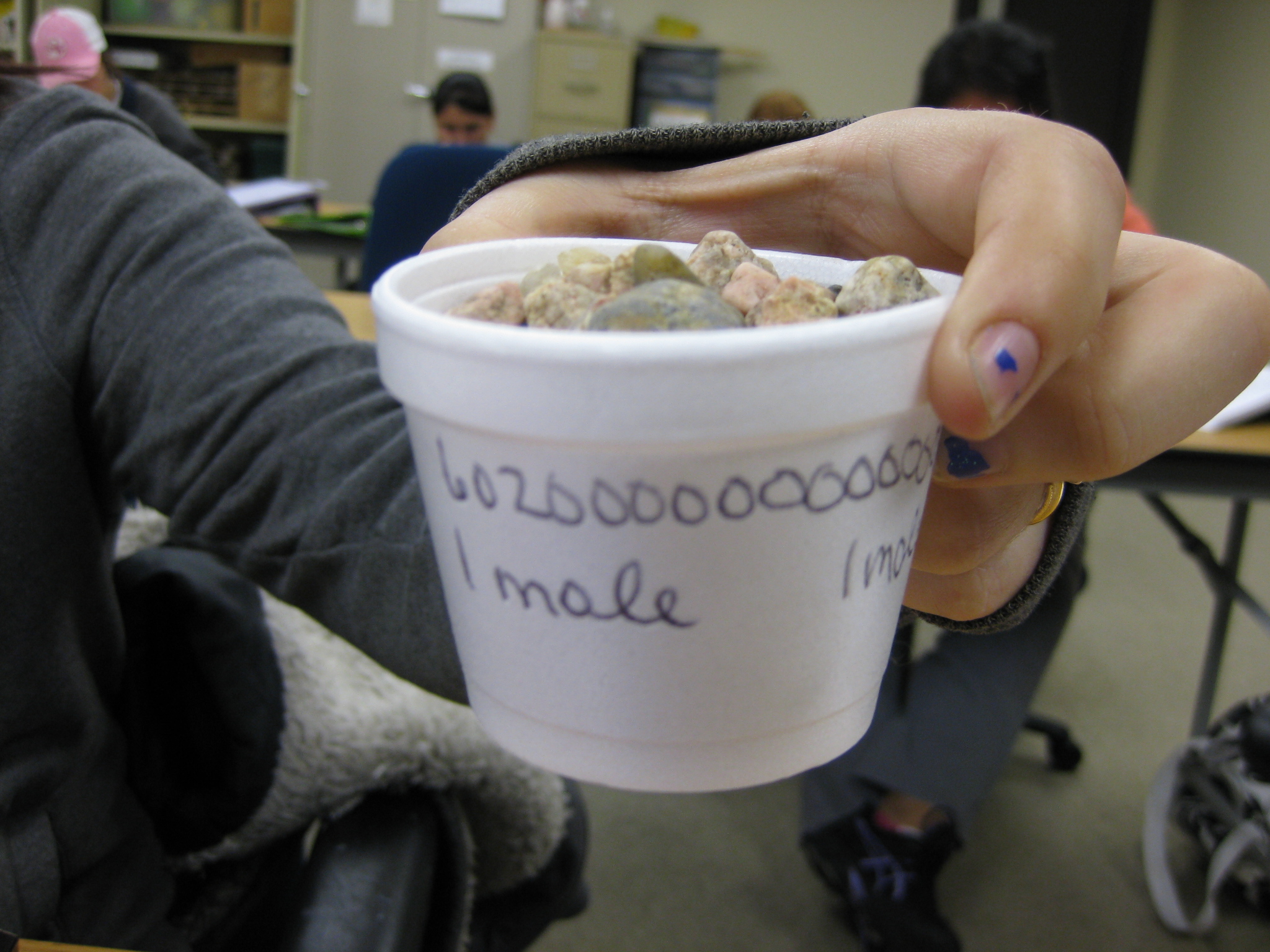

"Lead" "Aluminuim"
Ask the same question regarding the aluminum (cotton ball cup.) Emphasize again that while they have one mole of both "lead" and "aluminum" in their cups and that while the mass is greatly different, the number of atoms present is the SAME (6.02 x 1023 atoms)!!
At this time, I go back to the periodic table and ask them to tell weights of one mole of various elements. From this point, we move to the practice pages in the lesson.
-------------------------------------------------------------------------------------------------------------------------------------------------
Do you have ideas and photos to share with other Friendly Chemistry users? Send them to us at our email address below and we'll post them for others to see.
Questions? Email us at friendlychemistryinfo@gmail.com For more information about Friendly Chemistry, visit www.friendlychemistry.com